About Us
One of the pioneers of medical device production in Turkey
Ertunç Özcan Ithalat and Mumessillik were established in Ankara in 1968. Intensive care units, simulation centers, emergency units, operating room-anesthesia devices, intensive care - sterilization service and key delivery hospital projects are avaliable as a solution partner.

Strong Team Growing and getting stronger every day since 1968 we contribute to the country's economy and employment.
Employment Our aim is to employ people who have adopted the prin development and development and the effectiveness of employees, to ensure the continuation of their happiness and success
Our Quality Standards Our productions ISO 9001:2015 and ISO 13485:2016 Standards.
Safe Our factory has the Facility Security Certificate issued by the Ministry of National Defense.
Our Mission
Mission Statement
At Ertunc Ozcan, we are committed to advancing global healthcare quality with innovative and superior medical device manufacturing. Our goal is to offer healthcare professionals and patients state-of-the-art, dependable, and secure medical devices that elevate diagnostics, treatment, and patient outcomes.
Innovation
We constantly challenge technology and design limits to develop medical devices that meet the ever-changing demands of the healthcare industry. We cultivate a culture of creativity and urge our teams to experiment with new ideas.
Quality
We are dedicated to maintaining the highest quality standards in all our operations. Our exacting quality control procedures guarantee that our products always comply with or surpass regulatory requirements and industry norms.
Patient-Centric
We prioritize the well-being and comfort of patients in every product we develop. We strive to uphold ethical standards in all our practices and decisions, ensuring patient safety and well-being. Our devices are designed to improve patients' lives, accessibility to healthcare, and the patient experience.
Collaboration
We work closely with healthcare professionals, researchers, and partners to gain insights into the evolving healthcare landscape. By fostering collaboration, we can develop solutions that effectively address complex medical challenges.
Ethical Responsibility
We at the company uphold the highest ethical standards in all of our business practices. Our decision-making is guided by integrity, transparency, and accountability, ensuring the safety and trust of our customers and stakeholders.
Environmental stewardship is of utmost importance to us. We are committed to reducing our environmental footprint by developing eco-friendly manufacturing processes and sustainable products that reduce waste and energy consumption.
Employee well-being is at the forefront of our priorities. Our success is driven by our talented and dedicated team. We prioritize the well-being, growth, and development of our employees by creating an inclusive and supportive workplace where everyone can thrive.
Our vision is to become a global leader in medical device manufacturing, known for our relentless pursuit of innovation, commitment to quality, and dedication to improving patient care. We aim to make significant contributions to healthcare advancements, positively impacting the lives of millions of people worldwide.
Our vision is to become a global leader in medical device manufacturing, known for our relentless pursuit of innovation, commitment to quality, and dedication to improving patient care. We aim to make significant contributions to healthcare advancements, positively impacting the lives of millions of people worldwide.
• Code of Ethics.
• Product Security Statement.
Product Security Statement
This paper summarizes the Ertunc Ozcan position on securing our products, services, applications, and systems and describes our processes for providing products with Security Designed In.
Background
We at Ertunc Ozcan recognize that the security of Ertunc Ozcan NICU products and services are an important part of our security planning. We are dedicated to helping you maintain the confidentiality, integrity, and availability of personal data, business data, and the Ertunc Ozcan hardware and software products that create and manage this data.
Threats to the security of devices and personal and healthcare information continue to increase. These threats include malicious security attacks via viruses, worms, and hacker intrusions.
To fulfill our commitment to security, we at Ertunc Ozcan maintain a cyber security program to: develop, deploy, and support advanced security features for our products and services.
Manage security events in the field. Ertunc Ozcan participates in industry and government collaborations to help ensure product innovations and clinical information is produced and available at the highest level of quality, availability, and confidentiality.
We implement security within a heavily regulated medical device industry. Government regulations (e.g., those of the US Food and Drug Administration (FDA), European Commission) require that hardware and software changes be subjected to rigorous verification and validation to ensure high safety and performance standards are met in all Ertunc Ozcan medical devices.
Likewise, Ertunc Ozcan strives to ensure that same high standard for NICU products and services. Organization Ertunc Ozcan operates under a Product Security Policy governing Security Designed In creation of products and services.
The policy also governs risk assessment, identification of vulnerabilities in existing products, and incident response activities.
Technicial Specifications
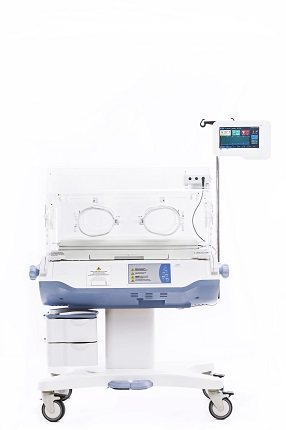
Technical Specifications of the Magic Loggia Ultimate M Infant Incubator
Microenvironment Control:
Precise temperature control: +/- 0.1°C accuracy.Adjustable humidity settings:20% to 95% relative humidity.
Integrated servo-controlled heating system.
Dual temperature probes for accurate monitoring and safety redundancy.
Access and Visibility
Transparent and easily accessible canopy design.Swiveling canopy with assisted lifting mechanism for effortless access to the infant.
Integrated grommet for medical procedures and minimal disturbance.
Safety and Monitoring
Continuous temperature and humidity monitoring with audible and visual alarms.Oxygen saturation (SpO2), Non-invasive blood pressure (NIBP), Perfusion index, heart rate , scale and oxygen monitoring capabilities.
Independent air filtration system to maintain a clean environment.
Closed-loop incubator system for minimized heat loss.
Ergonomic Design
Adjustable height for medical professionals' convenience.360-degree swivel with lockable positions.
Easy-to-clean, antimicrobial surfaces.
Customer Access Portal
Submit your Product Service Request here. An Ertunç Özcan Service Representative will respond within as soon as possible
Contact Us

Ertunç Özcan Sağlık Tesisleri ve Tıbbi Cihazlar İnşaat San. Tic. A.Ş.
Head Office : Aso 2. ve 3. Organize Sanayi Bölgesi Alcı (OSB) Mah. 2036 Cad. No:1 Sincan-Temelli-ANKARA
contact@ertuncozcan.com
Phone: +90 312 641 41 34 - Fax: +90 312 641 40 06